Description
GLOBAL SALES IN JAPANESE JEN , THE PRODUCT IS NOT FOR THE SERBIAN MARKET


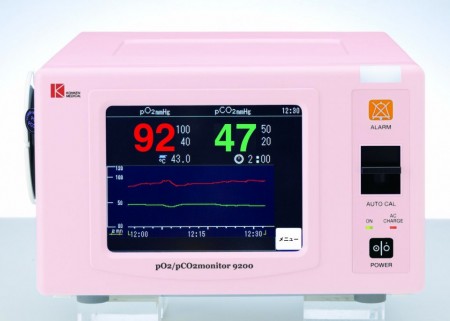
PCO2 iP-9200 monitor-Japan:
Klasifikacija klase Upravljanje medicinskim uređajima / Specifično održavanje Medicinskih
uređaja koji upravljaju održavanjem
Broj sertifikata za medicinske uređaje 221AGBZKS00307000
Showing a trend on your monitor screen
Capturing trends on screen can be selected from 4 ranges of 15 minutes, 30 minutes, 1 hour and 2
hours on one screen.
In any range, up to 4 ranges can be retroactively displayed on the screen. For example, when a
two-hour trend is set, a trend trend of up to 8 hours can be confirmed on the screen
Switch to pCO2 single mode
The popular pCO2 single mode in the 9000 series can be set easily in steps of 0.5 ° C from 37 ° C to 42 ° C.
Displays the calibration progress on the monitor screen
Displays the progress of the most frequently requested calibration on the screen. Since the progress of calibration can be seen at a
glance, the end of calibration can be easily predicted.
Product Specifications:
Measurement / display range Transcutaneous oxygen partial pressure pO2: 0 to 999mmHg (orange) Transcutaneous carbon dioxide partial pressure pCO2: 1 to 199mmHg (green) Message: Alarm / operation instruction Trend graph: Real-time measurement value
Alarm measurement range Transcutaneous oxygen partial pressure pO2 Upper limit: 0 to 999 mmHg Lower limit: 0 to 999 mmHg Transcutaneous carbon dioxide partial pressure pCO2 Upper limit: 1 to 199 mmHg Lower limit: 1 to 199 mmHg
pO2 / pCO2 sensor temperature 37 ℃-45 ℃ (in 0.5 ℃ increments)
Calibration Built-in automatic calibration Two-point automatic calibration with standard gas
External output Analog: 0 to 1000mV / 0 to 150mmHg Digital: RS232C
Built-in battery Dedicated battery: 7.2V Operating time: Approximately 45 minutes
Electrical rating AC100V 50 / 60Hz, 40VA or less
Classification of equipment against electric shock Type of protection: Class I equipment Degree of protection: Type BF
Dimensions 240 (W) x 3000 (D) x 149 mm (H)
Quality 4.8 kg (including built-in battery, but excluding calibration gas cylinder and sensor)
Class Classification Managed Medical Device / Specific Maintenance Managed Medical Device
Medical device certification number 221AGBZX00307000
JMDN code 36346000
INFORMATION for Global Customers
“We’ve been able to handle sales in Asia, North America, and Europe, etc., but we avoid the Middle East, Serbia and Africa regions because we don’t understand the local business customs and the import regulations, etc. are complicated.”
“We’ve received an order from a one-time deal for a shipment to a different region from the usual destination. Also, given that the product we’re selling has special requirements, we’re concerned about whether we’ll be able to find a reliable route.

Produktangaben
Gleichartige Produkte